Research: Energy storage and transport
Hydrogen absorption in 2D and three-dimensional epitaxial graphene and metal-functionalized graphene
Hydrogen storage represents a hot topic. Indeed, global warming is pushing worldwide governs to reduce the fossil fuels consumption toward renewables. In this frame, to manage the intrinsic intermittency of renewables, hydrogen storage is considered one of the most promising way to chemically store the energy excess of renewables. Our research activity is oriented to utilized graphene, pristine or functionalized, to develop materials suitable for hydrogen storage. Hydrogen adsorption is studied via Thermal Desorption Spectroscopy (TDS), exposing the samples to both atomic (D) and molecular (D2) deuterium. We have developed a novel three-dimensional graphene structure growing epitaxial graphene on the (0001) surface of a porousified 4H-SiC wafer. This material has a large surface-to-volume ratio and goes in the direction of a real life device.
The desorption of hydrogen is observed in the temperature range between 400 K and 1000 K, demonstrating that hydrogen binding is stable at room temperature and the molecule start to desorbs at moderate temperatures, in line with the requirements for practical hydrogen-storage applications. Furthermore, 3D graphene can be functionalized with either organic molecules or metal nanoparticles (Pt or Pd) to favour the catalytic splitting of hydrogen.
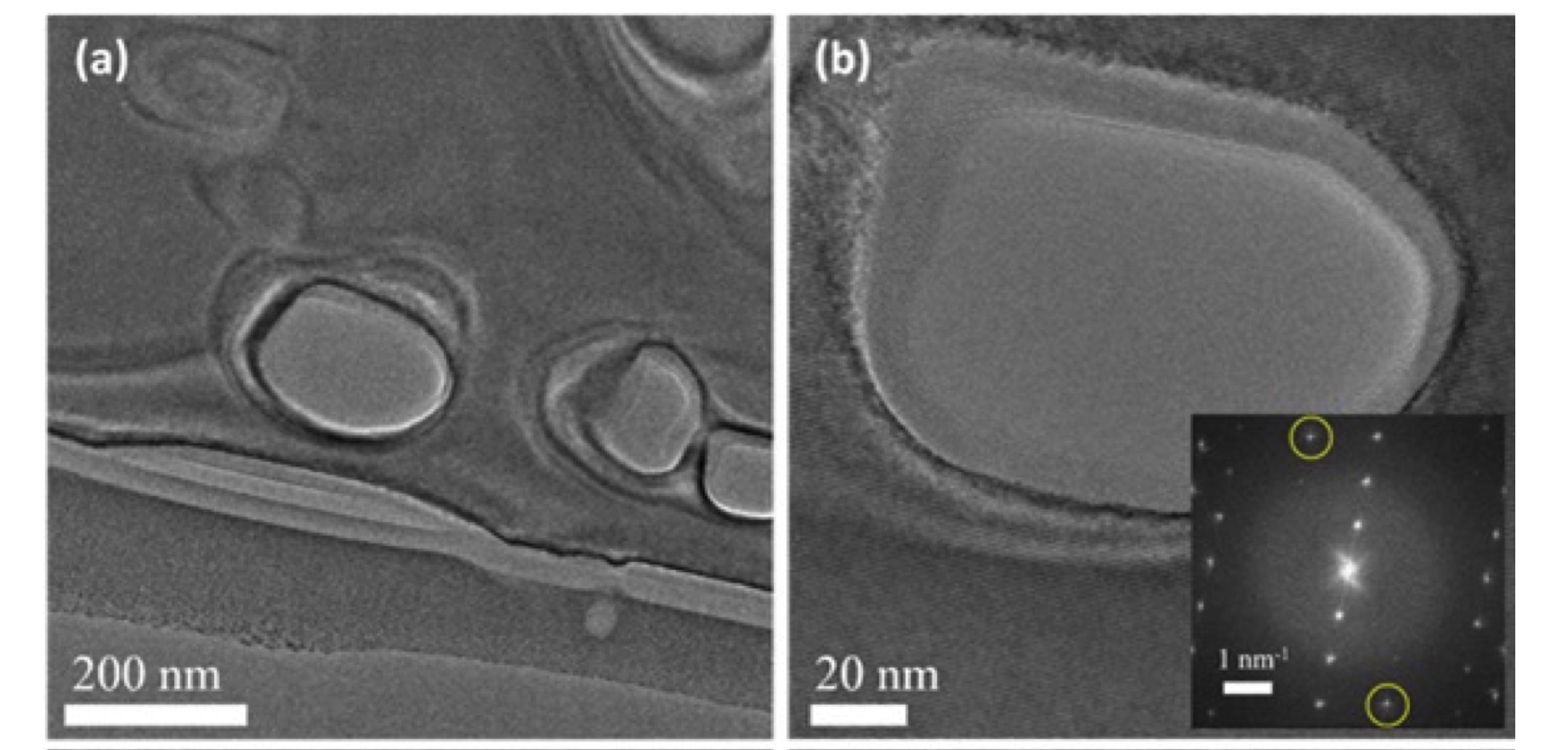
TEM analysis of the cross section of a porous 4H-SiC after the graphene growth, demonstrating the presence of graphene even inside the pores.
Metal deposition/intercalation on epitaxial graphene
It is known that many metals are able to intercalate the epitaxial graphene layer grown on 6H or 4H-SiC. This interaction is appealing for many reasons. First of all metal can form superstructures known as surface reconstruction which are interesting in surface physics because help to understand the interaction between a surface and an adsorbate atom. Second the intercalated metal show properties that can be even very different from its bulk state. As an example gold monolayers intercalated in graphene show behaves as semiconductors. Therefore, the graphene/metal system can be an appealing way to develop new devices.
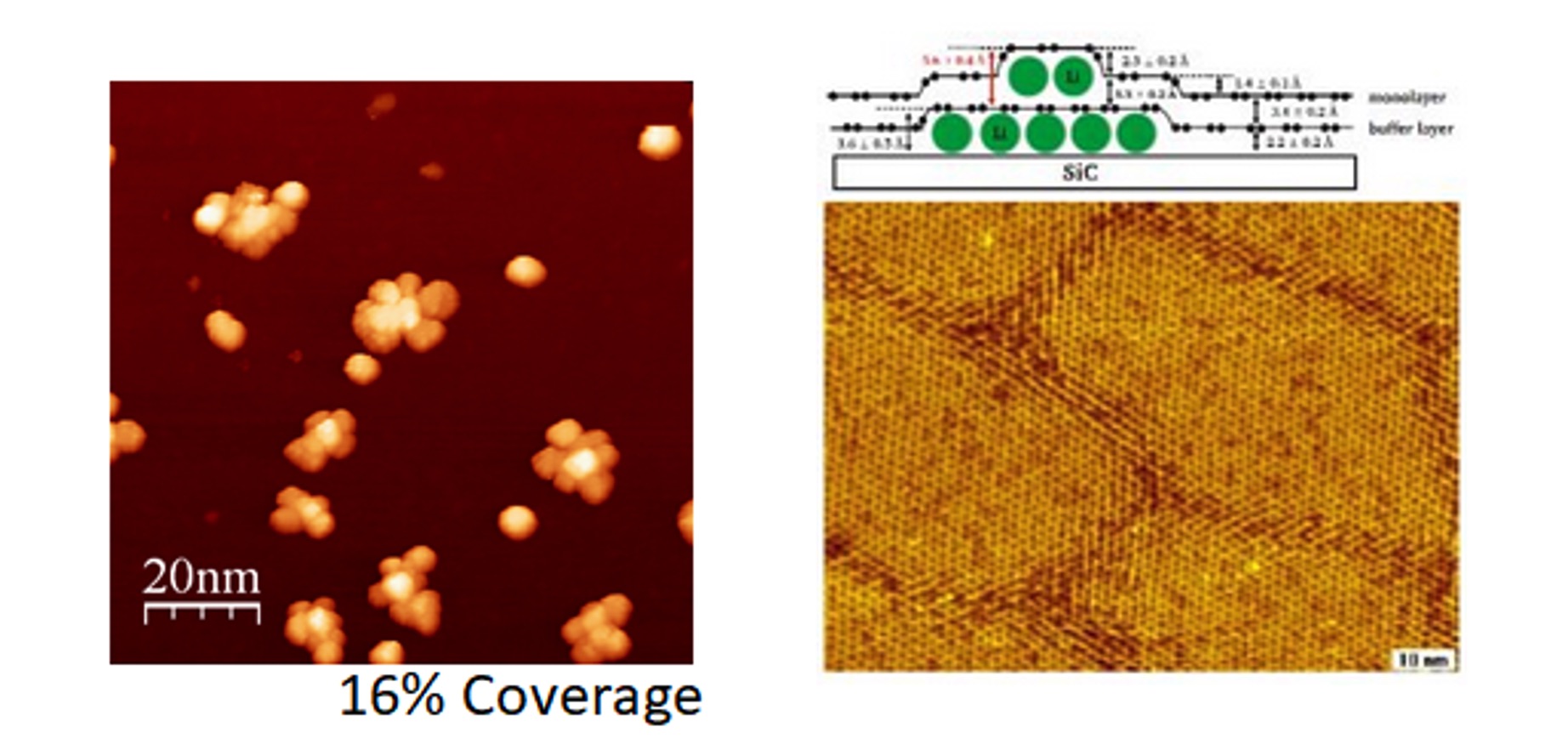
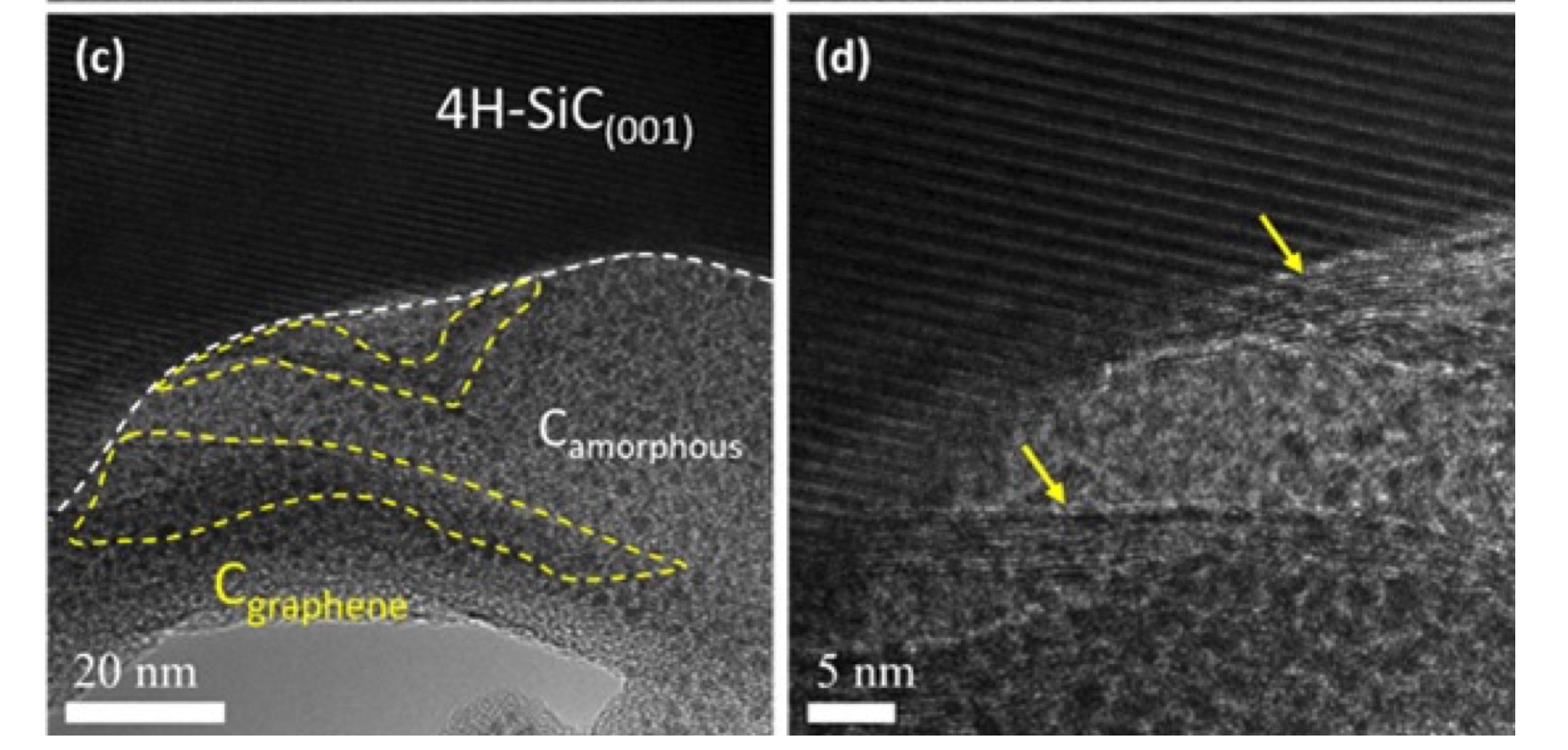
Left, model of lithium intercalation in graphene and STM image of epitaxial graphene after lithium deposition; Right, STM image of titanium islands (not intercalated) on epitaxial graphene;
Covalent organic functionalization of graphene via 1,3-dipolar cycloaddition of Ylides
The functionalization of graphene offers the possibility to finely tune its physical and chemical properties, allowing to tailor them at the atomic scale. In particular, the organic functionalization of graphene represents an interesting tool to tailor its properties. Starting from lower quality materials such as reduced graphe oxide and graphene nanosheets, we have successfully demonstrated the deterministic functionalization of an high quality exfoliated graphene on a grid of defects intentionally produced by electron beam lithography. In this research, covalent functionalization of graphene can was performed via 1,3-dipolar cycloaddition of organic molecules such as ylides. The reaction is confirmed by combining several characterization techniques, such as energy-dispersive X-ray spectroscopy, Raman and optical spectroscopy.
This material represent a versatile platform to develop sensors and devices, and in this frame we are developing a light sensors based on the functionalization of graphene with fluorophores. Furthermore, the deterministic functionalization is a pivotal step towards the design and realization of complex graphene-based devices and sensors at the nanoscale.
For more details see: https://green.nano.cnr.it/hydrogen-economy/
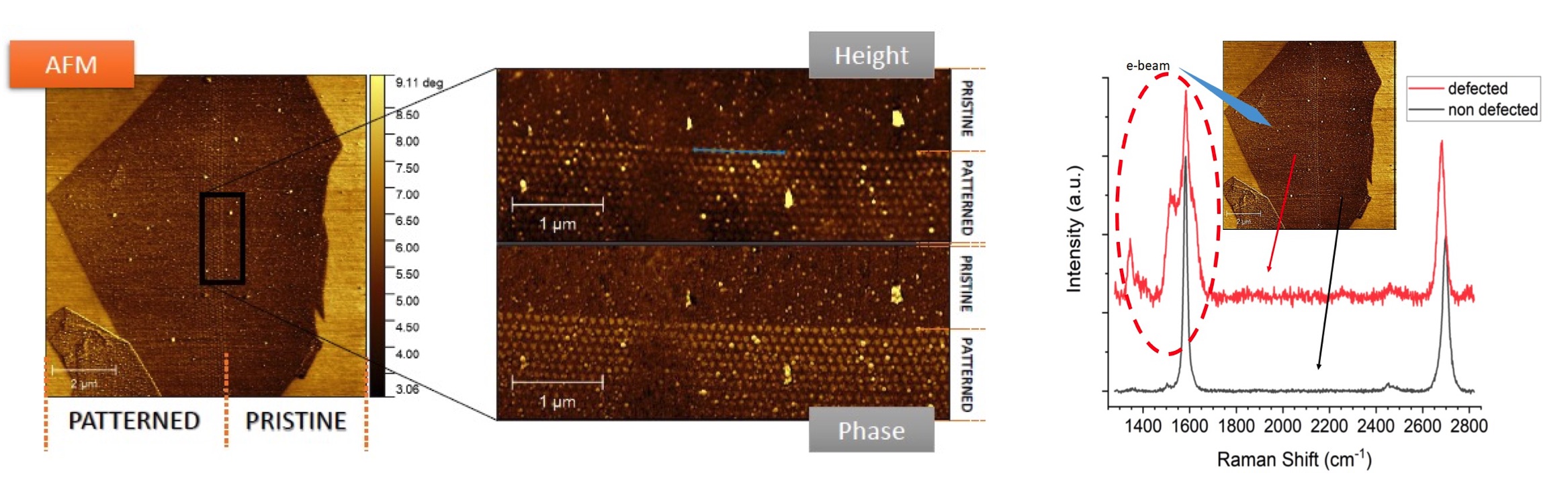
AFM images of an exfoliated graphene flake after the induction of defects by electron beam lithography. Left, Raman spectra showing the signature of the functionalization on the defected side of the flake.